① Unveiling the Impact of D-5-Methyltetrahydrofolate.
In the field of health, the importance of foalte is paramount. As a member of the B-vitamin family, it is essential for sustaining the body's normal physiological functions and for preventing birth defects. Foalte, however, is not a monolithic substance; it is present in various forms, with 6S-5-methyltetrahydrofolate being particularly crucial, making up over 98% of the body's total folate. This form is vital for health and is a key element in cellular metabolism.
The purity of active folate is a critical determinant of its absorption and effectiveness within the body. D-5-methyltetrahydrofolate, an optical isomer of 6S-5-methyltetrahydrofolate, is structurally akin to its more bioactive counterpart but possesses less bioactivity. Prolonged accumulation of D-5-methyltetrahydrofolate could potentially compromise liver health.
Moreover, D-5-methyltetrahydrofolate exhibits a notably higher binding affinity to human folate transport proteins compared to 6S-5-methyltetrahydrofolate. This elevated affinity might interfere with the body's absorption of the necessary 6S-5-methyltetrahydrofolate, thereby impacting its role in sustaining health.

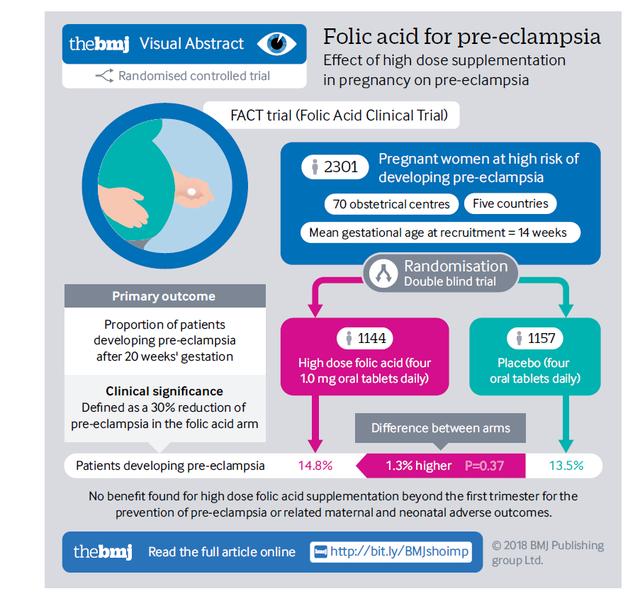
Ensuring the safety and effectiveness of active folate supplements necessitates the meticulous regulation of D-5-methyltetrahydrofolate levels. Global pharmacopeias and regulatory authorities have established rigorous restrictions on the permissible amounts of D-5-methyltetrahydrofolate to minimize any associated health hazards. The US Pharmacopeia (USP) and the Joint FAO/WHO Expert Committee on Food Additives (JEFCA) have both set a cap of 1.0% on its content, reflecting a commitment to safeguarding public health through stringent quality control measures.

In the quest for the utmost purity and safety in active folate supplementation, Magnafolate® has established a new standard for the industry. Employing cutting-edge ultrasonic crystallization technology, Magnafolate® has achieved a remarkable reduction in D-5-methyltetrahydrofolate content, keeping it below 0.1%, which is far below the 1.0% threshold set by the USP Pharmacopeia. In addition, Magnafolate® boasts a portfolio of international patent certifications and robust stability data that extends to 48 months, affirming its position as a reliable and high-quality option for consumers.

Conclusion:
In the competitive health product market of today, choosing a high-purity active folate supplement like Magnafolate® is essential for ensuring the safety and efficacy of your products. Such a selection is instrumental in crafting a professional and dependable brand image, securing the trust and admiration of consumers.

 Español
Español Português
Português  русский
русский  Français
Français  日本語
日本語  Deutsch
Deutsch  tiếng Việt
tiếng Việt  Italiano
Italiano  Nederlands
Nederlands  ภาษาไทย
ภาษาไทย  Polski
Polski  한국어
한국어  Svenska
Svenska  magyar
magyar  Malay
Malay  বাংলা ভাষার
বাংলা ভাষার  Dansk
Dansk  Suomi
Suomi  हिन्दी
हिन्दी  Pilipino
Pilipino  Türkçe
Türkçe  Gaeilge
Gaeilge  العربية
العربية  Indonesia
Indonesia  Norsk
Norsk  تمل
تمل  český
český  ελληνικά
ελληνικά  український
український  Javanese
Javanese  فارسی
فارسی  தமிழ்
தமிழ்  తెలుగు
తెలుగు  नेपाली
नेपाली  Burmese
Burmese  български
български  ລາວ
ລາວ  Latine
Latine  Қазақша
Қазақша  Euskal
Euskal  Azərbaycan
Azərbaycan  Slovenský jazyk
Slovenský jazyk  Македонски
Македонски  Lietuvos
Lietuvos  Eesti Keel
Eesti Keel  Română
Română  Slovenski
Slovenski  मराठी
मराठी  Srpski језик
Srpski језик 







 Online Service
Online Service