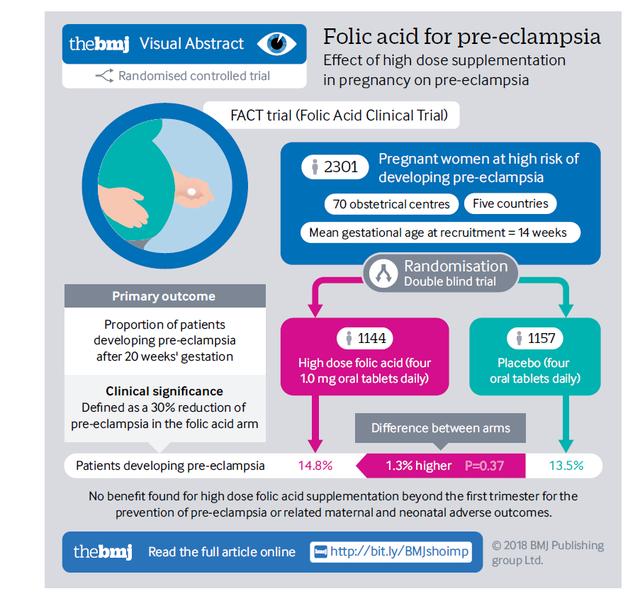
Folic acid is essential for the health of pregnant women and their fetuses. As an active form of folate, (6S)-5-methyltetrahydrofolate glucosamine salt has been approved for use in several regions.
However, the Brazilian Ministry of Health and the National Health Surveillance Agency (ANVISA) have recently issued a specific risk warning about the use of (6S)-5-methyltetrahydrofolate glucosamine salt by pregnant women, which has caught the industry's attention and led to discussions.
Key Points of the Warning from Brazil's Ministry of Health and the National Health Surveillance Agency (ANVISA):
The Ministry of Health and ANVISA, after thorough analysis of current scientific research and with a strong commitment to public health, have specifically pointed out that pregnant women should take into account the limitations of current scientific research and assess their personal health conditions when considering the use of (6S)-5-Methyltetrahydrofolate Glucosamine Salt. This initiative is designed to ensure the safety of pregnant women and their fetuses and to caution consumers and medical professionals to carefully evaluate such products before making a choice.
Historical Regulatory Directives Review and Update:
In 2018, ANVISA issued Regulatory Directive No. 28, which for the first time addressed the risks associated with the use of certain folic acid supplements by pregnant women. The directive mandates that product labels must clearly state:
"The warning 'In pregnant women, it must be assessed whether the maternal condition justifies the potential risk to the fetus, considering (6S)-5-Methyltetrahydrofolate Glucosamine, as the evidence is very limited to determine the risk of this compound in pregnancy.' must be included on the product labeling. "


Regulatory Link:http://antigo.anvisa.gov.br/documents/10181/3898888/An%C3%A1lise+de+Contribui%C3%A7%C3%B5es+-+Ciclo+Discuss%C3%A3o+-+Suplementos+Alimentares/d3c135a6-6560-4f33-8c2d-09f29434bd34?version=1.0
In 2020, ANVISA issued Regulatory Directive No. 76, reaffirming the warning content on product labels to ensure that consumers are clearly informed and can weigh the risks of use.
"The warning 'In pregnant women, it must be assessed whether the maternal condition justifies the potential risk to the fetus, considering (6S)-5-Methyltetrahydrofolate Glucosamine, as the evidence is very limited to determine the risk of this compound in pregnancy.' must be included on the product labeling. "


Regulatory Link:http://antigo.anvisa.gov.br/documents/10181/5809185/IN_76_2020_.pdf/dfd37f9a-678f-4d04-86e7-d44a8ee9490b
Conclusion:
The measure taken by Brazil not only serves as a safeguard for its consumers but also sets an important precedent for the use of folate supplements globally. It highlights that different regions may have varying recommendations for the same product due to differences in regulatory policies, the evolution of scientific research, and the diverse needs of public health. This serves as a reminder that when selecting and using any nutritional supplement, it is essential to consider regional differences and adhere to the guidance of local regulatory authorities.
This report is intended to convey the risk warning issued by Brazil's Ministry of Health and ANVISA regarding the use of (6S)-5-Methyltetrahydrofolate Glucosamine Salt by pregnant women. The information provided is for reference purposes only, and for further details, please visit the official website of ANVISA at anvisa.gov.br.

 Español
Español Português
Português  русский
русский  Français
Français  日本語
日本語  Deutsch
Deutsch  tiếng Việt
tiếng Việt  Italiano
Italiano  Nederlands
Nederlands  ภาษาไทย
ภาษาไทย  Polski
Polski  한국어
한국어  Svenska
Svenska  magyar
magyar  Malay
Malay  বাংলা ভাষার
বাংলা ভাষার  Dansk
Dansk  Suomi
Suomi  हिन्दी
हिन्दी  Pilipino
Pilipino  Türkçe
Türkçe  Gaeilge
Gaeilge  العربية
العربية  Indonesia
Indonesia  Norsk
Norsk  تمل
تمل  český
český  ελληνικά
ελληνικά  український
український  Javanese
Javanese  فارسی
فارسی  தமிழ்
தமிழ்  తెలుగు
తెలుగు  नेपाली
नेपाली  Burmese
Burmese  български
български  ລາວ
ລາວ  Latine
Latine  Қазақша
Қазақша  Euskal
Euskal  Azərbaycan
Azərbaycan  Slovenský jazyk
Slovenský jazyk  Македонски
Македонски  Lietuvos
Lietuvos  Eesti Keel
Eesti Keel  Română
Română  Slovenski
Slovenski  मराठी
मराठी  Srpski језик
Srpski језик 







 Online Service
Online Service