Introduction
Preeclampsia is a grave pregnancy complication that poses a significant threat to the health of expecting mothers worldwide. It is a contributing factor to premature births, increased perinatal health issues, mortality rates, and long-term disabilities. While the role of folic acid as a B vitamin in preventing neural tube defects is widely recognized, its potential to prevent preeclampsia, particularly in the later stages of pregnancy, has been a subject of keen medical interest. A landmark international multicenter trial published in The BMJ in 2018 shed new light on this question.

Research Background
Affecting about 3-5% of all pregnancies globally, preeclampsia is a leading cause of maternal deaths. With limited treatment options available—delivery being the only definitive cure—the search for effective preventative measures is of paramount importance. As a widely endorsed vitamin supplement, folic acid has sparked considerable research interest, particularly regarding its potential benefits in the second and third trimesters of pregnancy.

Trial Design
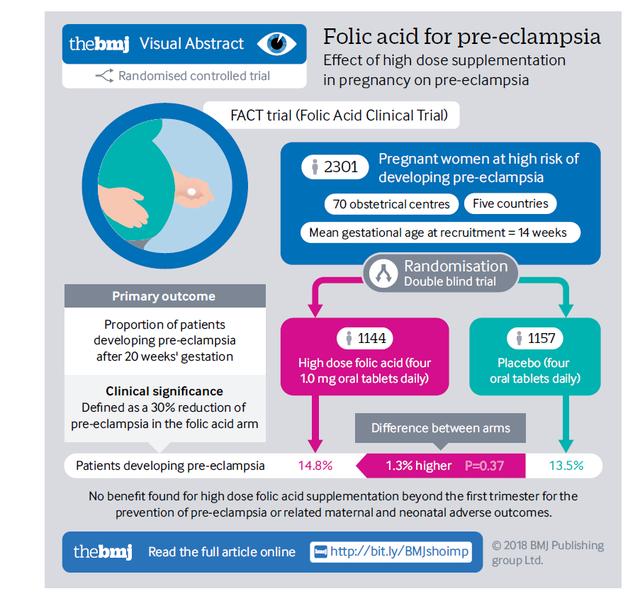
Dubbed the "FACT" trial, this investigation aimed to assess the efficacy of high-dose folic acid in preventing preeclampsia among pregnant women identified as being at higher risk. This double-blind, Phase III, randomized controlled trial was conducted across multiple international centers in Argentina, Australia, Canada, Jamaica, and the United Kingdom. A total of 2,301 eligible pregnant women, identified as being at high risk for preeclampsia, were randomly assigned to either a high-dose folic acid group (receiving four 1.0 mg oral tablets daily) or a placebo group from the 8th to the 16th week of gestation until delivery.

Main Results
The primary outcome measured was the incidence of preeclampsia. The study found that 14.8% of women in the folic acid group developed preeclampsia, compared with 13.5% in the placebo group—a difference that was not statistically significant (relative risk 1.10, 95% confidence interval 0.90 to 1.34, P=0.37). No significant differences were observed between the two groups in terms of other adverse maternal or neonatal outcomes.

Research Significance
The findings from the FACT study carry profound implications for public health policy. They indicate that high-dose folic acid supplementation beyond the first trimester is not an effective strategy for preventing preeclampsia in high-risk women. This revelation suggests that existing recommendations regarding folic acid supplementation may warrant reevaluation and adjustment.

Research Direction Outlook
Although folic acid did not demonstrate the anticipated preventative effects against preeclampsia, researchers remain undeterred. This finding has, in fact, ignited further research into strategies for the prevention of pregnancy complications. Looking ahead, there is a growing anticipation for the development of innovative approaches to more effectively reduce the incidence of preeclampsia and safeguard the health of both expecting mothers and newborns.
References:
Wen SW, White RR, Rybak N, Gaudet LM, Robson S, Hague W, Simms-Stewart D, Carroli G, Smith G, Fraser WD, Wells G, Davidge ST, Kingdom J, Coyle D, Fergusson D, Corsi DJ, Champagne J, Sabri E, Ramsay T, Mol BWJ, Oudijk MA, Walker MC. Effect of high dose folic acid supplementation in pregnancy on pre-eclampsia (FACT): double blind, phase III, randomised controlled, international, multicentre trial. BMJ 2018;362:k3478. doi:10.1136/bmj.k3478.

 Español
Español Português
Português  русский
русский  Français
Français  日本語
日本語  Deutsch
Deutsch  tiếng Việt
tiếng Việt  Italiano
Italiano  Nederlands
Nederlands  ภาษาไทย
ภาษาไทย  Polski
Polski  한국어
한국어  Svenska
Svenska  magyar
magyar  Malay
Malay  বাংলা ভাষার
বাংলা ভাষার  Dansk
Dansk  Suomi
Suomi  हिन्दी
हिन्दी  Pilipino
Pilipino  Türkçe
Türkçe  Gaeilge
Gaeilge  العربية
العربية  Indonesia
Indonesia  Norsk
Norsk  تمل
تمل  český
český  ελληνικά
ελληνικά  український
український  Javanese
Javanese  فارسی
فارسی  தமிழ்
தமிழ்  తెలుగు
తెలుగు  नेपाली
नेपाली  Burmese
Burmese  български
български  ລາວ
ລາວ  Latine
Latine  Қазақша
Қазақша  Euskal
Euskal  Azərbaycan
Azərbaycan  Slovenský jazyk
Slovenský jazyk  Македонски
Македонски  Lietuvos
Lietuvos  Eesti Keel
Eesti Keel  Română
Română  Slovenski
Slovenski  मराठी
मराठी  Srpski језик
Srpski језик 







 Online Service
Online Service