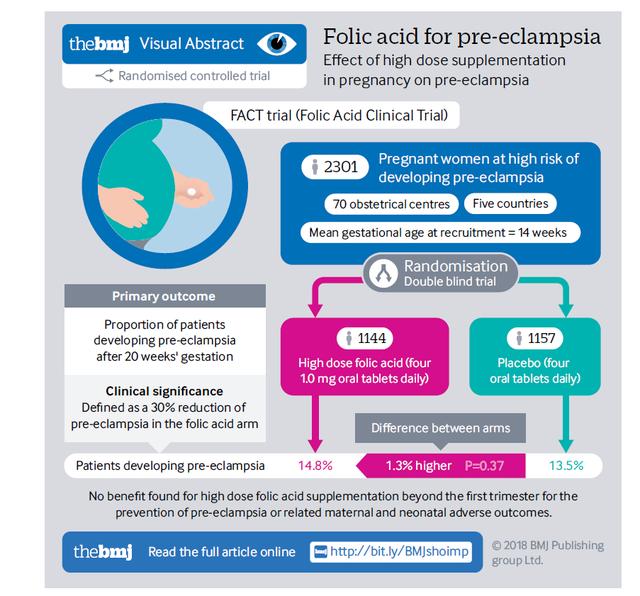
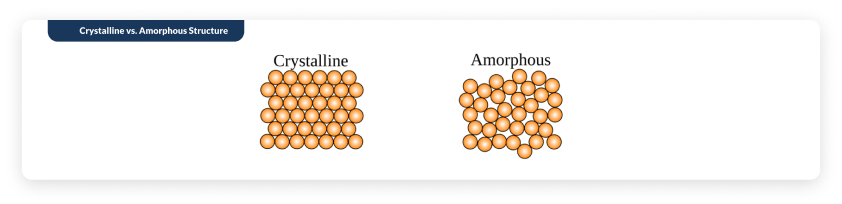
Have you heard of crystalline vs. amorphous types? Most of us don’t know the difference between the two, but in the biochemistry world, it makes a really BIG difference to stability.
Crystalline salt molecules are considered extremely stable whereas amorphous salt molecules are known to be much less stable because of the higher exposure to oxygen. Check out the picture below showing a representation of the structure differences. It makes it easy to see why the crystalline molecule would be more stable than the amorphous (in the holes is oxygen - and therefore more oxidation and destabilization).
With the unique C crystal structure, Magnafolate can well isolate oxygen, so it can be stable for 3 years at the room temperature. Actually, great stability for Magnafolate also means much purer because it is not easier to degrade and bring harmful impurities.

 English
English Español
Español  Português
Português  русский
русский  Français
Français  日本語
日本語  Deutsch
Deutsch  tiếng Việt
tiếng Việt  Italiano
Italiano  Nederlands
Nederlands  ภาษาไทย
ภาษาไทย  Polski
Polski  한국어
한국어  Svenska
Svenska  magyar
magyar  Malay
Malay  বাংলা ভাষার
বাংলা ভাষার  Dansk
Dansk  Suomi
Suomi  हिन्दी
हिन्दी  Pilipino
Pilipino  Türkçe
Türkçe  Gaeilge
Gaeilge  العربية
العربية  Indonesia
Indonesia  Norsk
Norsk  تمل
تمل  český
český  ελληνικά
ελληνικά  український
український  Javanese
Javanese  فارسی
فارسی  தமிழ்
தமிழ்  తెలుగు
తెలుగు  नेपाली
नेपाली  Burmese
Burmese  български
български  ລາວ
ລາວ  Latine
Latine  Қазақша
Қазақша  Euskal
Euskal  Azərbaycan
Azərbaycan  Slovenský jazyk
Slovenský jazyk  Македонски
Македонски  Lietuvos
Lietuvos  Eesti Keel
Eesti Keel  Română
Română  Slovenski
Slovenski  मराठी
मराठी  Srpski језик
Srpski језик 







 Online Service
Online Service