Introduction
6S-5-Methyltetrahydrofolate (6S-5-MTHF) is a metabolic product of folic acid in the body, accounting for more than 98% of the total folate in the human body. Compared to synthetic folic acid, 6S-5-MTHF can be directly absorbed in the body without being limited by dihydrofolate reductase (DHFR) and 5,10-methylenetetrahydrofolate reductase(MTHFR). It can rapidly increase the levels of serum folate and red blood cell folate, and it does not mask vitamin B12 deficiency, making it a revolutionary upgrade and substitute for synthetic folic acid.
However, the stability of 6S-5-MTHF is a significant challenge. It is highly prone to degradation, which can result in the formation of numerous impurities. These include compounds such as JK12A, (6R)-Mefoxc, (6S)-Mefox, Tetrahydrofolic acid, 7,8-Dihydrofolic acid, 5,10-Methylenete-trahydrofolic acid, 5-Methyltetrahydropteroic acid and Dimethylte-trahydrofolic acid. The presence of these impurities can impact the purity and effectiveness of folate supplements.
5-Methyltetrahydropteroic acid
5-Methyltetrahydropteroic acid is a common impurity of 6S-5-Methyltetrahydrofolate (6S-5-MTHF). Upon oxidation, this impurity transforms into the compound with the structure known as JK1303.
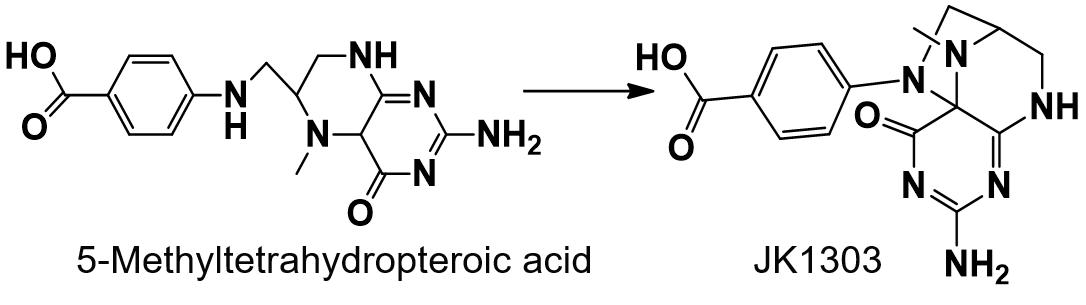
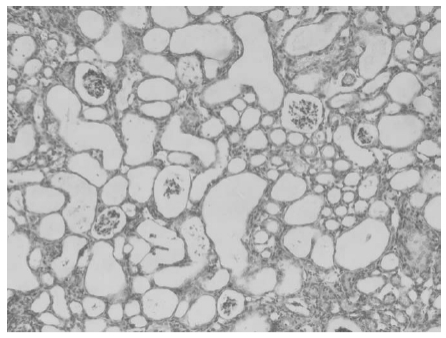
Compound JK1303 exhibits nephrotoxic effects, including renal tubular necrosis and an elevated kidney index. It may also indirectly impact urine metabolism, leading to increased levels of serum transaminases.
Research has shown that at a dosage of 500 mg/kg, compound JK1303 can already induce death and a reduction in weight gain in mice, with toxicity becoming more pronounced at higher doses. When mice are administered a single dose of JK1303 at 1000 mg/kg, severe renal tubular necrosis is observed after 14 days, and autopsy findings reveal a significant number of vacuoles in the renal tubules.
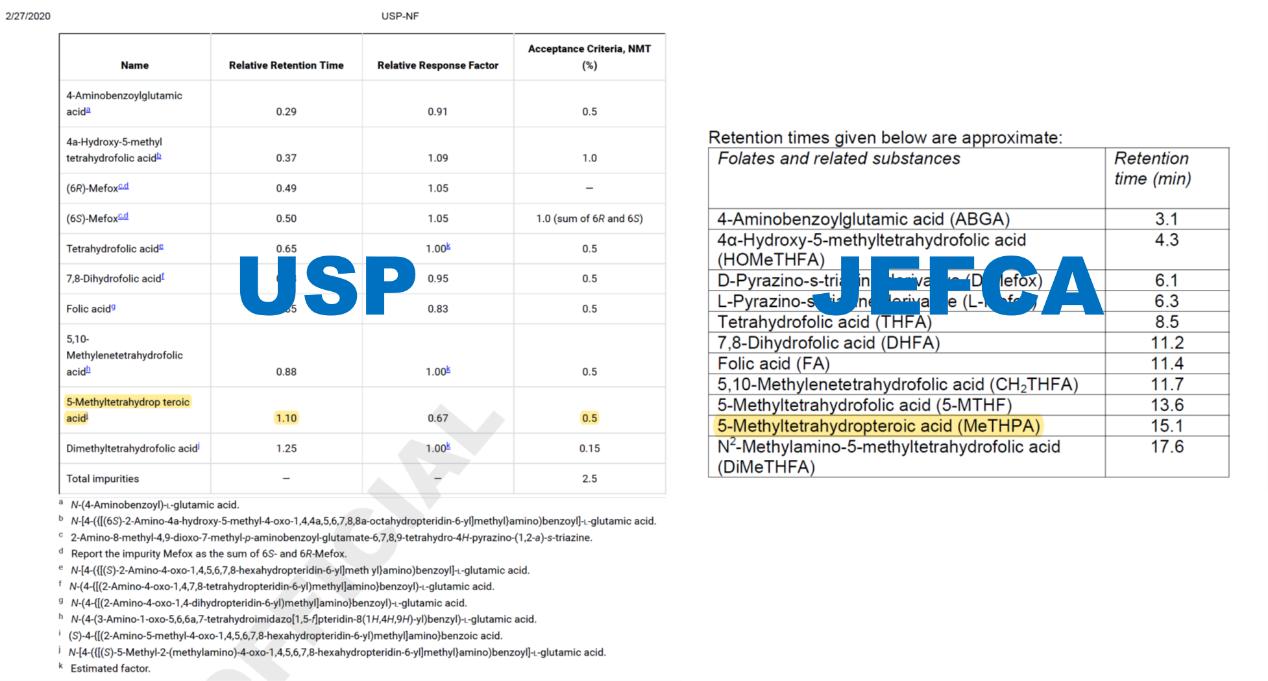
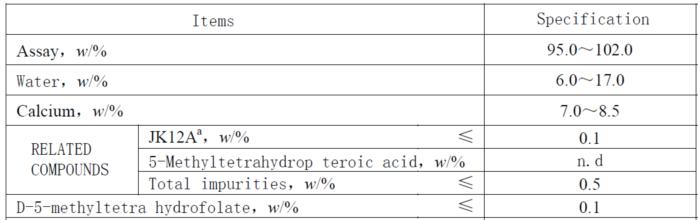
Control of 5-Methyltetrahydropteroic acid
Ensuring the safety and effectiveness of active folate supplements necessitates the meticulous regulation of 5-Methyltetrahydropteroic acid levels. Global pharmacopeias and regulatory authorities have established rigorous restrictions on the permissible amounts of 5-Methyltetrahydropteroic acid to minimize any associated health hazards. The US Pharmacopeia (USP) and the Joint FAO/WHO Expert Committee on Food Additives (JEFCA) have both set a cap of 0.5% on its content, reflecting a commitment to safeguarding public health through stringent quality control measures.
Magnafolate®
In the quest for the utmost purity and safety in active folate supplementation, Magnafolate® has established a new standard for the industry. Employing cutting-edge ultrasonic crystallization technology, Magnafolate® has achieved a remarkable reduction in 5-Methyltetrahydropteroic acid content, keeping it below 0.05%(not detect), which is far below the 0.5% threshold set by the USP Pharmacopeia. In addition, Magnafolate® boasts a portfolio of international patent certifications and robust stability data that extends to 48 months, affirming its position as a reliable and high-quality option for consumers.
Conclusion
In the competitive health product market of today, choosing a high-purity active folate supplement like Magnafolate® is essential for ensuring the safety and efficacy of your products. Such a selection is instrumental in crafting a professional and dependable brand image, securing the trust and admiration of consumers.

 Español
Español Português
Português  русский
русский  Français
Français  日本語
日本語  Deutsch
Deutsch  tiếng Việt
tiếng Việt  Italiano
Italiano  Nederlands
Nederlands  ภาษาไทย
ภาษาไทย  Polski
Polski  한국어
한국어  Svenska
Svenska  magyar
magyar  Malay
Malay  বাংলা ভাষার
বাংলা ভাষার  Dansk
Dansk  Suomi
Suomi  हिन्दी
हिन्दी  Pilipino
Pilipino  Türkçe
Türkçe  Gaeilge
Gaeilge  العربية
العربية  Indonesia
Indonesia  Norsk
Norsk  تمل
تمل  český
český  ελληνικά
ελληνικά  український
український  Javanese
Javanese  فارسی
فارسی  தமிழ்
தமிழ்  తెలుగు
తెలుగు  नेपाली
नेपाली  Burmese
Burmese  български
български  ລາວ
ລາວ  Latine
Latine  Қазақша
Қазақша  Euskal
Euskal  Azərbaycan
Azərbaycan  Slovenský jazyk
Slovenský jazyk  Македонски
Македонски  Lietuvos
Lietuvos  Eesti Keel
Eesti Keel  Română
Română  Slovenski
Slovenski  मराठी
मराठी  Srpski језик
Srpski језик 







 Online Service
Online Service